
Small intestinal bacterial overgrowth (SIBO) is classified according to the types of gasses produced by microflora occupying the upper gut. The two types of gases are hydrogen, and methane. However, a new classification has emerged based on relatively recent findings which is characterized by excess hydrogen sulfide production. Hydrogen sulfide-dominant SIBO is now becoming recognized an independent entity, and may be very common in people who have been diagnosed with SIBO. In a previous article, I described the process of hydrogen sulfide metabolism in detail and presented the reasons for why this might occur as a beneficial adaptation in the context of glyphosate poisoning.
Hydrogen sulfide is a gas known for its characteristic sulfur-like odor. People passing gas that smells like rotten egg or cabbage are very familiar with this. This gas is mostly produced as part of the metabolism of a specific set of bacteria referred to as "sulfate-reducing bacteria", although it can be synthesized by many other bacteria and is also part of normal human metabolism in small amounts.

Sulfate-reducing bacteria metabolize hydrogen in conjunction with dietary sulfur sources (sulfite, sulfate, or other organic forms) and convert them into hydrogen sulfide gas. One of these types of bacteria which has been well-studies in the past is Desulfovibrio.
Hydrogen sulfide is established as a potent signalling molecule with both beneficial and detrimental effects on cells, depending on the type of cell and the concentration of the gas. In elevated concentrations, one of the ways hydrogen sulfide exerts its toxic effects is through its interaction with heme-containing proteins, causing severe cytochrome c oxidase deficiency and mitochondrial dysfunction.

Its transport is passive, meaning that the gas can freely travel throughout the blood stream and diffuse directly into cells through the cell membrane. Although hydrogen sulfide can exert pro-inflammatory effects on cells, it can also be fed through into the mitochondria to be used converted into energy (ATP) and the bioactive form of sulfur which cells can actually utilize (sulfate). Sulfate is needed for a wide variety of things including conjugation and detoxification, blood flow, and structural support. The conversion of hydrogen sulfide into sulfate involves multiple different steps performed by enzymes located in the mitochondria which include:
Sulfide-Quinone-Oxidoreductase
Thioreductase
Sulfur-Dioxygenase
Sulfite Oxidase
The initial step is catalyzed by sulfide-quinone-oxidoreductase, which serves to oxidase hydrogen sulfide by using ubiquinone (the oxidized form of CoQ) as an electron acceptor. CoQ deficiency has been shown to dramatically reduce SQR expression, inhibiting the cell's ability to break down hydrogen sulfide. (). Animal research shows that CoQ deficiency leads to the intracellular accumulation of hydrogen sulfide, alterations in downstream enzyme activity, and depleted glutathione levels. However, the researchers also found that supplementation with CoQ10 successfully restored SQR levels back to normal.

This suggests that supplementation with CoQ10 may therefore be an unexpectedly useful adjunct in a protocol designed to support healthy sulfur metabolism.
Side note: Many protocols used to treat problems with sulfur metabolism are aimed at restricting sulfur-containing foods, such as the amino acids cysteine and methionine. However, restriction of sulfur amino acids has actually been shown to increase hydrogen sulfide synthesis via upregulated cystathionine γ‐lyase, along with a concomitant decrease in the levels of glutathione. Therefore, dietary sulfur restriction is likely not a beneficial solution in the long-term.
A later step in the process of sulfide metabolism involves the conversion of sulfite, a cytotoxic metabolic intermediate, into sulfate. As you can seen above, the enzyme responsible for this is called sulfite oxidase (SUOX). SUOX is dependent on molybdenum as a cofactor, and in the event of reduced sulfite oxidase activity (due to genetics or cofactor deficiency), a high influx of hydrogen sulfide into the cell may result in a buildup of intracellular sulfite.
Elevated sulfite poses many potential risks for the cell, which include damage to proteins, lipids, and mitochondrial dysfunction. However, the point I would like to focus on next is the effects it has on thiamine.
Enter: Thiamine
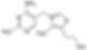
Thiamine (vitamin B1) is a critical component of glucose, amino acid, and lipid metabolism. It is also needed for the synthesis of NAPH, nucleic acids, and neurotransmitters, along with facilitating nerve transmission. High concentrations are found in pork, organ meats, fortified grains, and (high sources), although there is a small amount in most other foods.
Thiamine is particularly susceptible to degradation by thiaminase enzymes found in certain foods such as raw fish, the polyphenols in coffee and tea, along with metabolic byproducts of mold such as Aspergillus. Metabolism of refined sugars and carbohydrates also raises the requirement for thiamine by increasing the flow of glucose through glycolysis.
Although host gut bacteria are responsible for synthesizing a large quantity of thiamine and may contribute significantly toward total thiamine intake, there are also groups of bacteria including Clostridia and Bacillus which produce thiaminases and can destroy thiamine. It is therefore possible that severe cases of dysbiosis could have a severe impact thiamine status. Long-term gastrointestinal malabsorption frequently accompanies dysbiosis and SIBO, and is another factor which has been implicated in thiamine deficiency.
It is also possible that thiamine status is negatively affected in hydrogen-sulfide dominant SIBO. Although the animal research investigating hydrogen sulfide has yielded inconclusive results, it is well established that sulfite (an intermediate in hydrogen sulfide metabolism) can destroy thiamine. The sulfite ion is capable of cleaving thiamine at its methylene bridge, rendering the vitamin ineffective. Hence, because hydrogen sulfide influx into the cell may result in higher sulfite concentrations, this could place intracellular thiamine stores at risk for degradation.
In support of this, in vitro data shows that sulfide reduces the concentration of thiamine in white blood cells. Furthermore, veterinary data shows that excess dietary sulfur coupled with a subsequent rise in hydrogen sulfide production increases the requirement for thiamine in the brain and central nervous system and can eventually lead to secondary thiamine deficiency. Additionally, thiamine supplementation was shown to protect animals against sulfide toxicity, which suggests that excess hydrogen sulfide does indeed negatively impact the integrity of thiamine.
Interestingly, clinical reports have shown that people diagnosed with SIBO are more likely to be deficient in thiamine. Since thiamine is absorbed via the gastrointestinal tract, maldigestion and malabsorption likely contribute to this finding. However, it is also possible that this could relate to the defects in sulfide metabolism described above.
How to test for thiamine
Based on the above information, it may be prudent for anyone experiencing digestive issues to test for functional thiamine status. Unfortunately, this is not as simple as just measuring blood or plasma thiamine. According to Dr Derrick Lonsdale, a world-leading expert on thiamine, blood thiamine concentrations provide an inaccurate measurement of thiamine status inside the cell and are only reflective of recent thiamine intake. Likewise, urinary excretion of thiamine is not a good measure and will not necessarily detect insufficiency.
On the other hand, functional sufficiency can sometimes be detected by measuring erythrocyte transketolase activity. Since tranketolase requires thiamine, low transketolase activity can indicate poor intracellular availability of thiamine. However, Dr Lonsdale's experience showed that transketolase testing alone was not always sufficient, and that it could remain somewhat high despite inadequate thiamine levels. As a solution, a "thiamine pyrophosphate effect" test used in conjunction with tranketolase activity could provide a lot more information. Thiamine pyrophosphate effect testing measures how readily thiamine is uptaken by cells. A high pyrophosphate effect indicates that cells need a lot of thiamine and so are likely deficient, whereas a low pyrophosphate effect suggests that cells already have sufficient amounts.
Transketolase activity testing is provided by a laboratory in the UK called Biolab and can be purchased cheaply, so that is one option. However, the thiamine pyrophosphate effect test is not currently offered by any lab at the time of writing this article. Another method for testing thiamine status also exists with reportedly very high sensitivity. It measures whole-blood thiamine pyrophosphate, which is reported to correlate strongly with intracellular thiamine concentrations and provide a more accurate representation of thiamine status. This test is currently offered by LabCorp.

Functional markers providing an indication of thiamine status can also be picked up on urinary organic acids, and plasma or urinary amino acids. Some of the markers to look out for include:
Elevated urinary pyruvate and/or lactate - Thiamine is a cofactor for the PDHC, an enzyme complex responsible for converting pyruvate into acetyl coA. In thiamine deficiency, PDHC may be inhibited and this can lead to a buildup of pyruvate, which may also be shunted toward lactate production. The result is higher levels of pyruvate and lactate.
Elevated alpha ketoglutarate/2oxoglutarate - Thiamine is a cofactor the KGDH, an enzyme complex involved in the TCA cycle responsible for converting alpha ketoglutarate into succinyl-coA. Low thiamine may reduce the rate of this reaction, resulting in higher levels of alpha ketoglutarate.
Elevated alanine - Pyruvate can be used to synthesize alanine, and alanine can also be converted back into pyruvate to be used in energy production. Again, the conversion of pyruvate requires adequate PDHC activity, and so thiamine deficiency can result in elevated alanine along with pyruvate.
Conclusion
Hopefully after reading this, the audience can appreciate how SIBO is particularly relevant to thiamine metabolism. The purpose of this article was to describe the how SIBO and gut-related issues can potentially lead to thiamine deficiency, and why people suffering from this condition would do well to test their thiamine status.
In the case long-term deficiency, thiamine repletion may actually go a long way toward fixing the initial problem. This is because thiamine deficiency might not only be a consequence, but also a cause of SIBO in the first place.
In the next article, I will be explaining how chronic thiamine deficiency can be causally linked with SIBO and other gut dysfunction.
